U.S.-based Acurion, led by CEO Rick Fultz—a seasoned healthcare executive recognized among San Diego’s 40 Under 40—is transforming global cancer diagnostics through AI-powered genomic insight.
The company joined K-Startup Grand Challenge (KSGC) 2025 Phase 2 to expand into Asia’s precision oncology landscape. The K-Startup Grand Challenge is Korea’s flagship government-backed accelerator helping global startups scale across Asia through access to funding, partners, and institutional support.
KoreaTechDesk spoke with Fultz about Acurion’s journey, the challenges of genomic testing, and how the KSGC program accelerated its Korea strategy.
Acurion Mission: Faster, Fairer Precision Oncology
Q1. What motivated you to start this company, and what core problem were you trying to solve?
Although I am not one of Acurion’s original scientific founders, I was recruited to lead the company as CEO because of my experience building and scaling businesses, as well as my longstanding relationships across the life sciences and healthcare sectors.
My primary motivation to join Acurion came from recognizing a clear global problem: patients wait far too long for genomic results that determine life-saving cancer treatments. Around the world, oncologists often depend on next-generation sequencing (NGS) tests that take three to five weeks, cost patients significant out-of-pocket fees, and frequently fail due to insufficient tissue or limited genetic coverage. In many markets, NGS is not even reliably available, forcing oncologists to make treatment decisions with incomplete data.
Even in advanced healthcare systems such as Korea, HRD and BRCA testing remain slow, expensive, and centralized. While HRD is the only actionable biomarker in several of the deadliest cancers, existing methods under-identify many eligible patients.
Acurion was created to change that. Using AI, we extract NGS-equivalent genomic insights directly from routine digital pathology slides within minutes. Validated in our JCO 2024 publication and across studies involving more than 1,200 patients, OncoGaze™ identifies two to three times more HRD-positive patients than existing NGS tests—without the delay. That mission—delivering faster, more equitable precision oncology—is what compelled me to lead and scale Acurion.
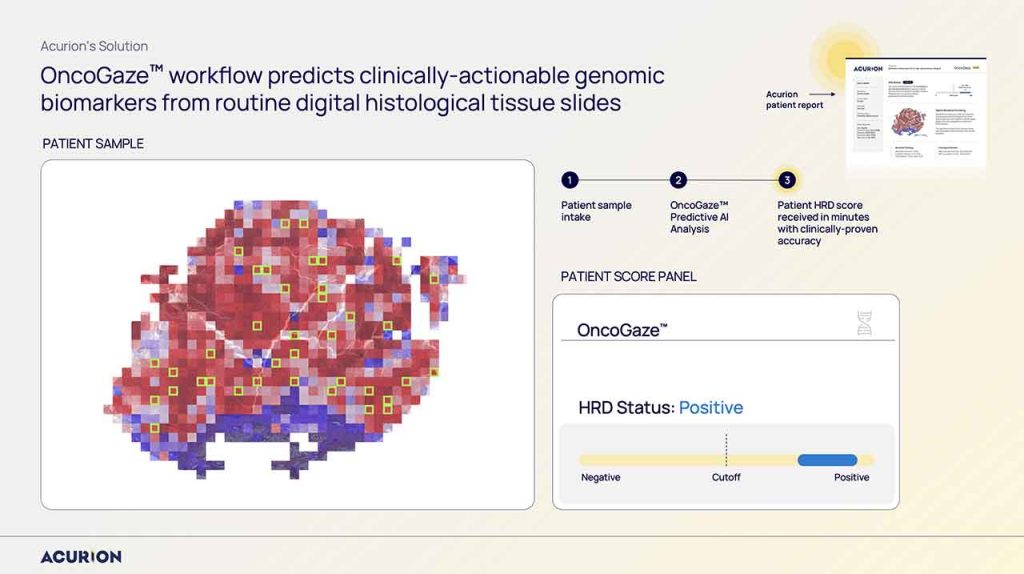
Identifying the Opportunity in Korea
Q2. What opportunity or unmet need did you identify in the Korean market, and what early signals convinced you that your solution could gain real traction here?
Korea is one of the world’s most advanced digital pathology markets, yet precision oncology still faces major bottlenecks from slow and costly NGS workflows. Many hospitals report two- to four-week turnaround times, constrained tissue availability, and BRCA-focused tests that miss up to half of HRD-positive patients—delays and gaps that directly affect therapy selection.
This issue is particularly critical for triple-negative breast cancer (TNBC), ovarian, and pancreatic cancers, which together account for a significant portion of Korea’s approximately 250,000 annual cancer cases.
The earliest and strongest signals of traction came directly from Korean doctors. In early 2025, we met with Korea University Medicine and CHA Hospital Center, where oncology leaders expressed strong interest in how OncoGaze™ could accelerate diagnosis, reduce reliance on centralized molecular labs, and enable faster treatment decisions.
Equally encouraging was the response from Korean investors. We entered due diligence with several prominent venture capital funds that recognized both the clinical value and commercial scalability of our platform. I also have longstanding relationships with Korean healthcare leaders across the Ministry of Health, GGP and Seoul government sectors, and major incubators—all of whom have been deeply supportive of Acurion’s market entry.
These early engagements validated that Korea represents not only a strong product-market fit but also the ideal early launch market for our precision oncology technology.
Insights That Reshaped Our Market Strategy
Q3. During KSGC, were there any mentors, partners, or specific insights that significantly influenced your product or strategy?
Yes. One of the most transformative insights came from clinical and regulatory mentors who emphasized that even world-class AI solutions struggle to gain traction if they disrupt existing workflows. Integration and localization are essential for adoption.
This guidance reshaped our roadmap to focus on seamless interoperability with the digital platforms already used by Korean hospitals and pathology labs. Discussions with KSGC-affiliated advisors and our Korea partner Flann also clarified the MFDS (Ministry of Food and Drug Safety) approval pathway, highlighting the importance of localized validation using Korean clinical and drug-trial datasets. These insights are now fully incorporated into our regulatory and deployment plans.
Equally important, the mentorship and administrative support from both KSGC and SparkLabs were invaluable for us as a young company entering Korea. Their hands-on advice on pursuing MOUs, establishing a local business entity, and identifying strategic partners has had a lasting impact on our market-entry approach.
We are deeply grateful to the KSGC and SparkLabs teams for shaping not only our Korea strategy but also our broader global approach to responsible AI deployment in healthcare.
Transformative Growth through KSGC
Q4. After joining KSGC, what has been the most meaningful change for your company, and what evidence supports this growth?
Since joining KSGC, Acurion has experienced transformative growth in partnerships, investment traction, and Korea strategy. The most meaningful change has been the acceleration of international partnerships—especially in Korea—and our progress toward commercialization readiness.
We established direct relationships with leading hospitals, investors, and government-linked organizations, including Korea University Medicine, SparkBioLabs, GBSA, and multiple venture groups such as Korea University Holdings, AJU IB, Yuanta, MobyDick, and Kakao Ventures. These collaborations are now laying the groundwork for Korean clinical pilots and helping shape our MFDS regulatory pathway.
KSGC also amplified our fundraising momentum. Our ongoing USD 6 million seed round (valued at a USD 12 million pre-money valuation) has secured USD 2.1 million committed, anchored by lead investor TK Partners (Thailand), whose chairman has joined our Board. This traction is supported by interest from more than 20 U.S. venture firms and several Korean funds.
Finally, KSGC enabled us to formalize our localization roadmap—initiating the establishment of a Korean subsidiary in 2026, exploring office sites in Suwon, Pangyo Techno Valley, and Gangnam, and aligning with regional partners to build a high-value local team.
Together, these milestones demonstrate measurable progress: strengthened institutional partnerships, deepening investor engagement, and a defined operational roadmap that positions Korea as one of Acurion’s first international launch markets.
Where Acurion is Heading Next
Q5. Looking ahead, what is the most important vision or long-term goal your company aims to achieve, and what steps are you taking to move toward it?
Acurion’s long-term vision is to make precision oncology globally accessible by transforming every routine digital biopsy into an immediate molecular test. Our goal is simple but ambitious: OncoGaze™ becomes as routine as the biopsy itself—delivering real-time genomic insights that guide therapy decisions for millions of cancer patients worldwide.
To achieve this, we are executing four key strategic pillars:
- Global Regulatory Clearances
We are pursuing coordinated regulatory approvals across multiple regions—the U.S. (MolDX and FDA 510(k) for pancreatic cancer), Korea (MFDS approval followed by NECA/HIRA reimbursement), and Brazil—with milestones extending through Q4 2026. - Scaled Clinical Validation
Our technology has already been validated in over 1,200 patients and published in JCO 2024, showing that OncoGaze™ identifies two to three times more HRD-positive patients than NGS while reducing turnaround time from weeks to minutes. Upcoming multi-center trials in Korea will strengthen regulatory submissions and accelerate adoption. - Strategic Expansion into Korea
Korea is set to become one of our earliest international launch markets. We are establishing a Korean subsidiary, building a Gyeonggi-based operations hub, and working closely with hospitals, investors, and government-linked partners to drive deployment and commercialization. - Platform Expansion
Beyond HRD, we are extending OncoGaze™ to detect additional clinically actionable biomarkers, broadening its clinical and therapeutic impact.
Our vision is a future where waiting weeks for genomic results is no longer acceptable—and where every patient receives the right treatment from day one.
Through K-Startup Grand Challenge 2025, Acurion demonstrates how AI innovation and international collaboration can make precision oncology faster, fairer, and more accessible—showcasing Korea’s vital role in shaping the global healthcare frontier.
“We are deeply grateful to the KSGC for our transformative growth across partnerships, investment traction, and Korea market strategy.”
About This Series
This article is part of the “K-Startup Grand Challenge 2025 Interview Series,” featuring 40 global startups from Phase 2 of Korea’s leading accelerator program. The series highlights how international founders are scaling innovation through Korea’s startup ecosystem.
Read more stories from the K-Startup Grand Challenge 2025 Interview Series on KoreaTechDesk.
🤝 Looking to connect with verified Korean companies building globally?
Explore curated company profiles and request direct introductions through beSUCCESS Connect.
– Stay Ahead in Korea’s Startup Scene –
Get real-time insights, funding updates, and policy shifts shaping Korea’s innovation ecosystem.
➡️ Follow KoreaTechDesk on LinkedIn, X (Twitter), Threads, Bluesky, Telegram, Facebook, and WhatsApp Channel.






