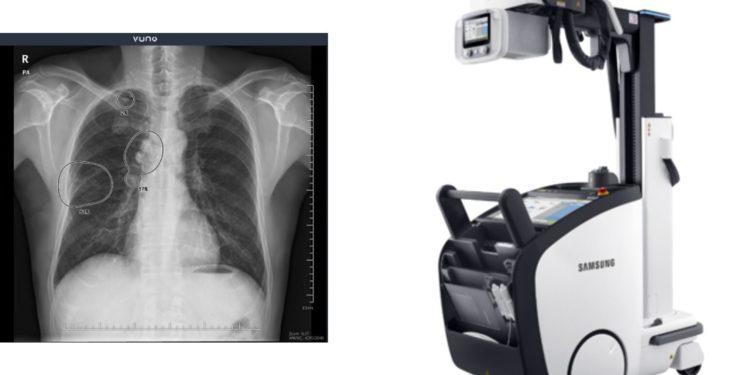
Korean AI-based Medical startup VUNO, which launched its IPO on February 26th, announced its partnership with Samsung Electronics Co., Ltd., a global medical device company. The MedTech startup will incorporate its AI-powered chest X-ray diagnostic solution, VUNO Med®-Chest X-ray™, into Samsung’s premium mobile digital X-ray system, GM85. The AI integrated X-ray suite is slated to debut in Korea and other major markets around the globe later in 2021.
VUNO AI solution to be embedded in Samsung’s premium Mobile X-Ray System
Samsung’s GM85, a premium mobile digital radiography system with a lightweight and ultra-compact design, combines a broad range of advanced technology, including a quick charging and long-lasting battery to enhance user convenience and superior image quality.
Featuring VUNO’s AI, the integrated mobile X-ray suite will be able to instantly deliver AI results on the spot upon scanning an image while the patient is still in the hospital. This has the potential to serve as a useful diagnostic support tool in the emergency room and intensive care units where real-time analysis is critical and under other medical environments with limited or no network connections.
“I am delighted to partner with Samsung Electronics in embedding our best-of-breed AI solution into their mobile digital X-ray devices. This collaboration will bring us closer to making our market-ready AI applications more accessible across the globe. We will continue to focus on advancing our technology to deliver improved patient outcomes,” said Hyun-jun Kim, co-founder and CEO of Seoul-based VUNO.
“Incorporating VUNO’s AI technology, we can introduce a more sophisticated mobile X-ray system with AI-enabled CAD,” said Woo-young Jang, Samsung Electronics’ Head of DR Business Team. “We will continue to extend our partnerships to develop a leadership position in the global X-ray market.”
VUNO Med®-Chest X-ray™ accurately and instantly detects and flags suspected chest abnormalities indicative of major pulmonary diseases such as tuberculosis, pneumonia, lung cancer based on five of the most common thoracic findings such as nodule/mass, pneumothorax, interstitial opacity, pleural effusion, and consolidation. This solution has been in full commercial deployment in Korea and Europe after obtaining MFDS (Ministry of Food and Drug Safety) approval and CE mark in August 2019 and June 2020, respectively.