Technology is bringing in many advanced changes to rehabilitation healthcare and therapeutic solutions. Korean startup, TechVillage, has developed a metaverse-based Remote VR Digital Treatment Solution for Prevention of Disorder of Senior Citizen and Restoration of the Lost Exercise and Cognition Functions of Patients with Brain Disorders (stroke, dementia, Parkinsonism, etc.)
Technology is bringing in many advanced changes to rehabilitation healthcare and therapeutic solutions. Korean startup, TechVillage, has developed a metaverse-based Remote VR Digital Treatment Solution for the Prevention of disorders of Senior citizens and Restoration of the Lost Exercise and Cognition Functions of Patients with Brain Disorders (stroke, dementia, Parkinsonism, etc.)
The biotech venture’s program that can assist rehabilitation patients has patients wearing a helmet-shaped head-mounted display. The helmet stimulates the use of the paralyzed parts of the body through various VR games, including hammering, catch-a-ball, cup pouring, touching bubbles, and playing a xylophone. The technology solution has been developed in collaboration with Seoul National University Hospital researchers.
The biotech venture’s program that can assist rehabilitation patients has patients wearing a helmet-shaped head-mounted display. The helmet stimulates the use of the paralyzed parts of the body through various VR games, including hammering, catch-a-ball, cup pouring, touching bubbles, and playing xylophone. The technology solution has been developed in collaboration with Seoul National University Hospital researchers.

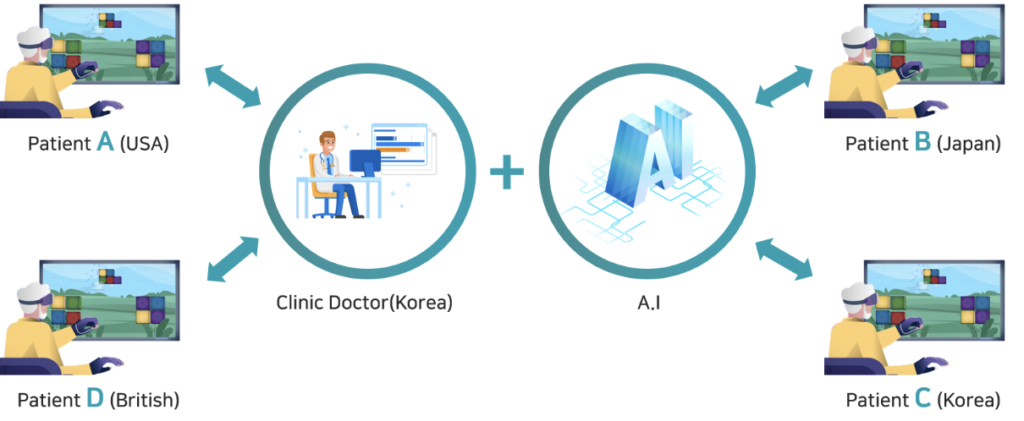
TechVillage’s solution is a complete immersive rehabilitation training and treatment solution. It allows remote treatment solutions where doctors from anywhere in the world can treat patients sitting in their homes. The company’s product has received official verification from Seoul National University Hospital, Chungnam National University Hospital and Dementia Center, Sosa Health Center, Bucheon City.
The company has also obtained approval for clinical trials of its digital medical device from the Ministry of Food and Drug Safety of Republic of Korea. The company expects to go through the process for US FDA approval as a digital therapeutic device next year.
The company has also obtained approval for clinical trials of its digital medical device from the Ministry of Food and Drug Safety of the Republic of Korea. The company expects to go through the process for US FDA approval as a digital therapeutic device next year.
The company wants to be a leader in manufacturing technology for digital therapeutical devices. With its differentiation and superiority compared to competitors’ products, TechVillage aspires for advanced market domination and global expansion.