While the South Korean government is fighting to control the spread of CORONAVIRUS (COVID-19) with the number of infections on the rise, the Korean business and science community is working to support the system to fight the effects of the virus. The latest number of infections in South Korea is now 8320 as of March 17, with 81 deaths and 1401 recovered cases.
Easy to use test-kits & drive-through test centres
A South Korean company has developed a tester that can detect the infectious virus in just 10 minutes. The company, PCL, is a provider of in vitro diagnostic products. The company said its breakthrough testing kit, named COVID-19 Ag GICA Rapid, can check nasal discharge for the presence of the virus within 10 minutes with an accuracy rate of around 85 percent.
The testing can be done at home, just like a regular pregnancy test, without people having to go to testing centers to have themselves checked if they suspect of having the virus. It also decreases the risk of exposure.
Currently, the Korean government is using RT-PCR to check COVID 19 infection. The said process is more accurate than COVID-19 Ag GICA, but it is time-consuming – six hours before a result is known – and can only be administered in hospitals or health centers.
PCL CEO Kim So-yeon was quoted as saying that the kit was developed using antibodies from China. A user can put nasal discharge into the kit and then get the result in 10 minutes. Kim said the antigen diagnostic kit would be a complementary measure for a quick test. “People infected with COVID 19 can spread the virus even at its earliest stage and at very mild symptoms, so the kit is a complementary test,” the company said.
In the city of Goyang, health workers in hazmat suits are stationed at parking lots for drive-through Corona19 testing on motorists. The authorities say it is a safer and quicker way for testing than in hospitals or health clinics.
Biotech Companies exporting Diagnostic Kits
Kogene Biotech, a biotech company has developed a kit that diagnoses Covid-19 using RT-PCR test method. The company is providing vital respiratory kits to authorities and organisations in Korea. Developers of Corona 19 diagnostic kits are also exporting test-kits to other countries.
Biotech company Seegene is sending its products to Germany and Italy. “We sent samples for product evaluation to Canada, Brazil, South Africa, Australia and Vietnam,” the company representative said. SolGent Co. Ltd, which received European CE certification on the 28th of last month, has signed export contracts with more than 20 countries including the Middle East, Europe and Southeast Asia. PCL, which recently received the RT-PCR diagnostic kit export license, is also negotiating an export contract.
Testing helping contain the spread and deaths
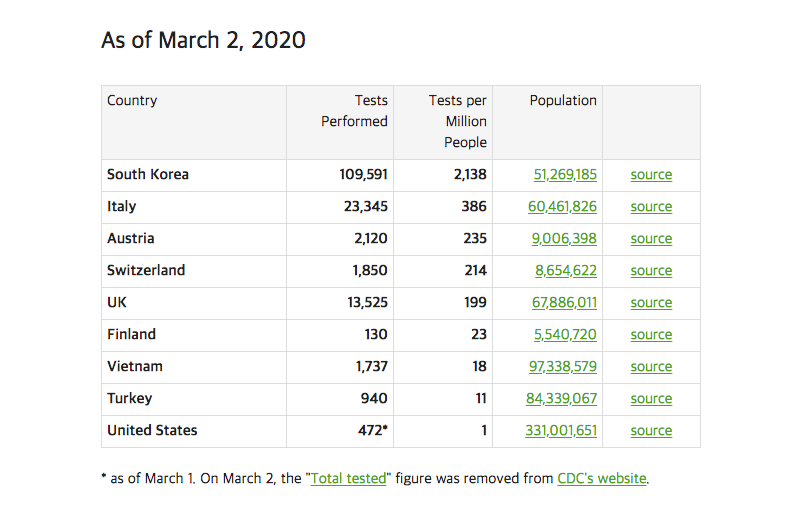
Though South Korea has seen the most cases of COVID-19 outside of China, the number of deaths compared to China are less. The Korean government and the medical industry is working hard to ensure the virus is not spread much outside Daegu, where it is found the most. The number of testing-kits and methods are saving the spread of the virus.
Emergency Drug-Trials for COVID-19 treatment
Pharmaceutical company Komipharm has filed for an emergency clinical trial for its investigative drug that could treat coronavirus pneumonia. This study is poised to become the first such study by a Korean company. The news about the drug trial made the stock prices of Komipharm rise on the Korean Exchange. Previously, Komipharm has succeeded in developing ‘Panaphix’, a clinical drug for inflammatory therapy for viral infections that can suppress the cytokine storm, and announced that it plans to carry out urgent clinical trials for patients with novel Coronavirus pneumonia.
It is known that Panapix has obtained the approval of the Korea Food and Drug Administration clinical trial plan to expand the application for Corona19. In the future, the company plans to provide emergency treatment for patients with Corona 19 pneumonia in Korea and third countries and to supply it to patients.
Another company, ImmuneMed, has announced that along with the Seoul National University Hospital (SNUH), it has got the government’s approval for Virus Suppressing Factor (VSF) as the treatment for new coronavirus patients, and are administering it on some subjects.
The Ministry of Food & Drug Safety has given its go-ahead to SNUH’s request for using ImmuneMed’s VSF on COVID-19 patients.
The two partners are planning to inject VSF four times — first, third, seventh, and 14th day — from the initial administration. The university, company and the ministry will jointly monitor the drug’s effects and side-effects after administering it.
A surge in innovative apps
There is a subsequent rise in the usage of smartphone apps by Koreans to detect the effects of Covid-19. Korean developers have developed apps using government data that allows users to check how close they have been to a confirmed COvid-19 patient. The app allows users to see the date a patient was confirmed with the disease, the demography of the patient, and some of their location history.
An app called Corona 100m launched on February 11, alerts a user if they have come within 100 m (328 ft) of a location visited by someone who’s had Covid-19. The apps have seen a tremendous surge in downloads, ranking among the week’s most-downloaded items in the country’s Google Play app store. Corona 100m has seen over 20,000 downloads per hour.
South Korea has become the country with the second-highest COVID-19 cases in the world outside China. The surge in the number of infections has impacted the Korean economy, and the government is fighting it with war foot measures.